Hello again fermentists,
It’s been a while, but I’m back in the writing mood. Today, let’s learn about how to effectively monitor beer quality from grain to glass. Whether you are a home brewer or a craft professional there are certainly many ways to assure quality of your brew. The three most important aspects of any brew are the pH during sugar extraction, the sugar content after brewing and the yeast cell count in the final fermentation vessel.
pH
The pH scale is essentially a measure of the concentration of free hydrogen ions in a solution. We typically think of pH as an acidity scale, where <7 is acidic and >7 is alkaline or basic. During brewing the effectiveness of the sugar extraction from grain during the mash is strongly influenced by the pH of the solution, with most enzymes converting longer sugars into shorter ones at a range of 5.2-5.6. Therefore, it is essential that brewers understand how pH can change during the mash and adjust it with food safe acids (lactic or phosphoric) and brewing salts (calcium chloride and calcium sulfate). To measure the pH of a solution a brewer must set up a calibrated pH meter. These don’t have to be crazy expensive and are usually nice little handheld units. To best illustrate this process, I thought I would share a standard operating procedure with pictures.
Standard Operating Procedure (SOP) for the measurement of beer, or wort pH
Purpose: pH is a proxy measurement for the acidity or alkalinity of a liquid solution. The relationship between beer pH, flavor and stability is well known. The pH of beer and wort need to be accurately measured from grain to glass to ensure proper enzyme conversion of malt sugars, as well as suitable growth conditions for yeast. A drop in pH below 3.5 during fermentation can mean a beer has been spoiled with lactic acid or acetic acid producing bacteria. This SOP is to take standardized pH readings of wort, or beer during brewing or from fermenters.
Frequency: At all stages of brewing, and fermentation.
Equipment needed:
Thermoworks handheld digital pH meter.
Polypropylene sampling bottle with lid for degassing wort or beer.
Black plastic caps for standard solutions.
White enamel cup for distilled water.
Grey spray bottles.
Stainless steel cups for cooling samples.
Materials needed:
Biopharm pH standard 7 (yellow bottle)
Biopharm pH standard 4 (pink bottle)
Distilled water
Peroxy acetic acid sanitizer at 200 ppm
Ice
Safety Information: This procedure is usually fairly safe. Samples taken from the brewhouse can be very hot, be careful not to burn yourself. Wear thermal heat resistant gloves if needed.
Safety Gear:
Thermal heat-resistant gloves
Nitrile disposable gloves
Safety Glasses
Water-proof boots
Procedure:
Spray out the zwickle sampling port with PAA. (If sampling wort during brewing, skip to step 4B.
Rinse out the PAA from the port into a polypropylene bottle and dump the solution.
Fill a bottle with 10 ml of sample.
Put cap on bottle and shake for 3-5 minutes to degas sample. Loosen the cap every minute to release gas. Wait until you can’t see many bubbles in the sample, or hear gas release when opening the cap of the bottle.
If you are sampling wort during brewing, no degassing is required. Instead you may need to cool the sample. Place the plastic bottle into a stainless steel cup filled with ice.
Take the pH meter out of the grey lunch box style container.
Calibrate the pH meter. This must be done every day before use. To calibrate:
Pour distilled water into white cup.
Pour standard 7 and 4 into two black caps. Do not stick pH probe directly into standard bottles as this could contaminate them.
Take the probe out of the electrode storage cap and place it into distilled water cup. Gently wick away water with kimwipe tissue.
Place the dry probe into pH 7 standard. Hold down cal button, until the display flashes. Once the reading is steady at 7-7.02, place probe into distilled water to rinse and dry as before.
Place the probe into the pH 4 standard. Once reading is steady at 4-4.03 press cal again.
The display will stop flashing and is now reading actual pH.
Example of calibrating a pH meter.
Place the probe into a sample to be tested.
Record pH in writing on brew sheet, or fermentation log.
After measurement, place the probe into distilled water, and if it is to be used again, leave in the cup. If you are done then gently wick away water and place the dry probe into an electrode storage solution. If the storage cap is dry, replace it with fresh electrode storage solution.
Turn off the pH meter and replace in case.
Troubleshooting: If the pH reading is not between 4 and 7, then the reading will not be accurate. A standard of 2 will need to be acquired if the pH is below 4. If the pH is above 7 then a standard of 10 will need to be acquired. If the sample is too warm or too cold the reading may not be accurate. The pH meter claims it is accurate at any temperature between freezing and boiling (34 -212 F), however it is best to take measurements of samples at or around room temperature (70 F). The probe has a thermometer (metal probe) that should be placed into all samples being tested so that the pH meter can accurately adjust the pH reading based on temperature.
Conclusion: pH readings are one of the quickest and most reliable measurements in the brewery and need to be documented accurately on a daily basis. If you are unsure of how to use the pH meter please read the user manual or reach out for help.
Sugar Concentration
To create tasty alcoholic drinks we first need yeast to consume sugars that we extract from grain. To be able to tell customers and consumers what type of alcohol by volume a beer has, we first need to know the starting and ending concentration of sugar in a solution. Now most homebrewers and probably even a lot of professional craft brewers are using a glass hydrometer to measure the dissolved sugars in their brews. However, I’m here to tell you there’s some better. The new Anton Paar EasyDensBluetooth digital hydrometer. I love this unit! It’s simple, fast, reliable and only uses a fraction of liquid compared to the traditional glass hydrometer. Below is my SOP for using this unit. Psst… Anton Paar give me free stuff!
Standard Operating Procedure for the measurement of gravity in beer, or wort
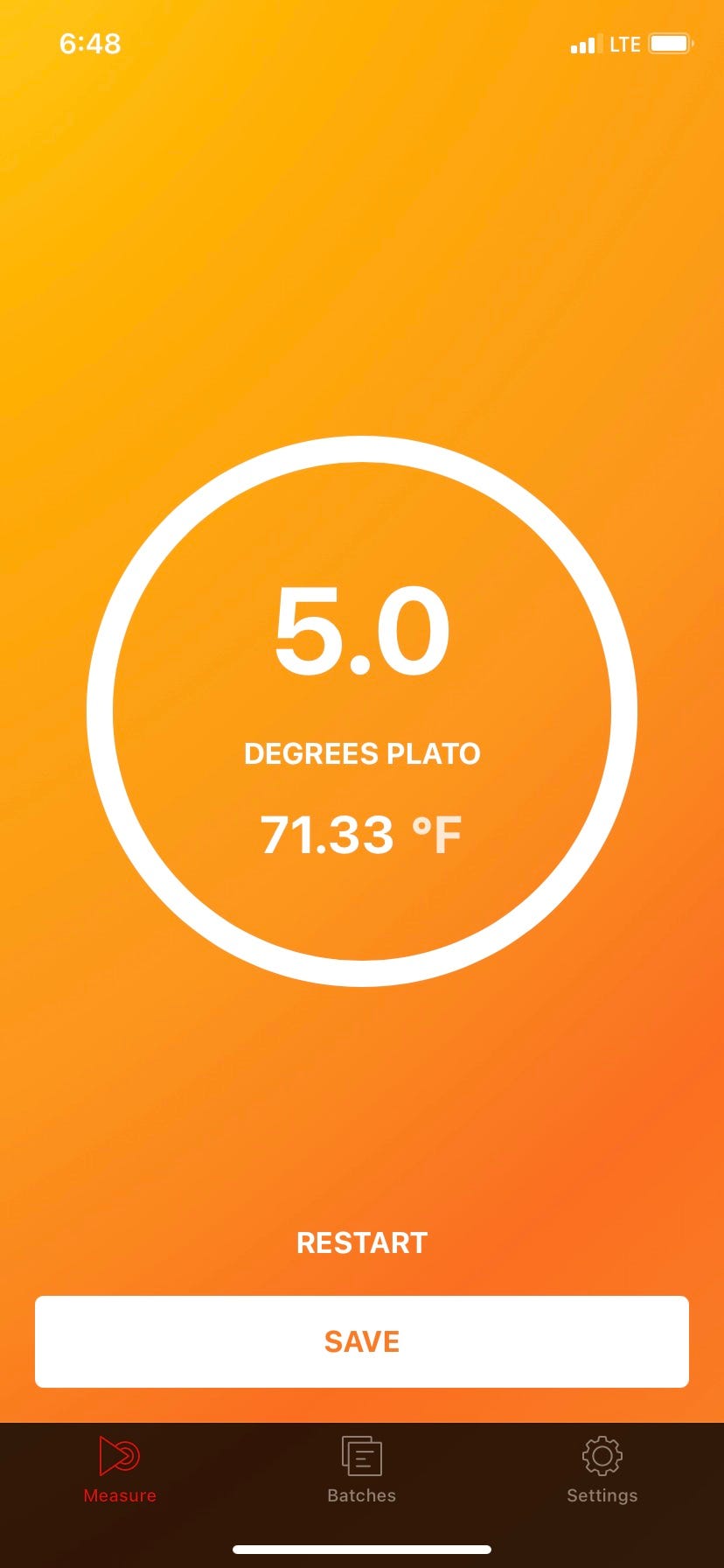
Purpose: Density is the measure of a mass of a liquid per volume. Degrees plato is a measurement of the concentration of dissolved solids in a brewery wort or beer. Degrees Plato (°P) is used to quantify the concentration of extract (mainly sugars derived from malt but also including other soluble material in wort) as a percentage by weight. A 10°P wort will contain 10 g of extract per 100 g of wort. The measurement of wort gravity is important to brewers in that it is an indicator of the potential alcoholic strength of the beer. As a very rough guide, every 1°P generates approximately 0.4% alcohol by volume—a 12°P wort will produce an average of approximately 5% alcohol by volume, depending on the extent to which sugars are fermented out. This SOP is to take a standardized measurement of sugar extract in degrees plato in beer and wort.
Frequency: At all stages of brewing, and fermentation.
Equipment needed:
Anton Paar Easy Dens Digital Density Meter.
Plastic syringe 12 mL.
Short poly tubing for waste disposal.
Polypropylene sampling bottle with lid for degassing wort or beer.
White enamel cup for distilled water.
Grey spray bottles.
Stainless steel cups for cooling samples.
Smartphone with bluetooth and BrewMeister App downloaded.
Materials needed:
Distilled water
Peroxy acetic acid sanitizer at 200 ppm
Ice
Safety Information: This procedure is usually fairly safe. Please be careful of hot wort when brewing. Wear thermal gloves if needed.
Safety Gear:
Thermal heat-resistant gloves
Nitrile disposable gloves
Safety Glasses
Water-proof boots
Procedure:
Spray out the zwickle sampling port with PAA. (If sampling wort during brewing, skip to step 4B.
Rinse out the PAA from the port into a polypropylene bottle and dump the solution.
Fill a bottle with 10 ml of sample.
Put cap on bottle and shake for 3-5 minutes to degas sample. Loosen the cap every minute to release gas. Wait until you can’t see many bubbles in the sample, or hear gas release when opening the cap of the bottle.
If you are sampling wort during brewing, no degassing is required. Instead you may need to cool the sample. Place the plastic bottle into a stainless steel cup filled with ice. Cool to about room temperature (70 F).
Take the Easy Dens unit out of its plastic cambro box above the microscope. Attach the poly waste hose to the right hole on the top of the unit and place the other end into a reservoir to collect waste.
Press the power button on the back of the unit. The unit will begin to flash lights. Open the Brewmeister app and turn on bluetooth on your smartphone. The unit will stop flashing once connected.
Example of my little brew lab setup with the EasyDens unit and pH meter.
Fill the syringe with your cooled, degassed sample by pulling up on the plunger. 2 mL is all that is required for each reading. Attach the syringe tip into the left hole on the unit.
Open the brewmeister app. Press start reading and push liquid through the syringe. The sample will flow out of the poly tube into your waste cup.
The reading will be over in seconds. Make sure no gas bubbles are present in the flow cell.
Record the data on the brew sheet or the fermentation log along with the temperature.
After all measurements, fill the syringe with distilled water and run the unit through to clean. Then pull the syringe with no liquid (just air) and push out any remaining liquid. Wipe any liquid from the top of the unit and place it back into its home cambro container.
Troubleshooting: If the sample is not degassed or too hot the reading will not be accurate. The easy dens will give you an error if the sample is too warm. If the unit is not pairing with your phone make sure your bluetooth is turned on and within the 30 feet range of the unit. A good measure to make sure the unit is working correctly is to run distilled water through and verifying that the degrees of plato are a big fat zero.
Conclusion: Plato gravity readings are the brewers best tool to monitor the progression of sugar extraction during brewing and the progression of fermentation. The final beer product needs to reach a terminal gravity that fits the style of that beer. If you are unsure of how to use the easy dens please read the user manual, and reach out help.
Yeast Counting
So, now you have a good handle on how to accurately determine the pH and the plato of your brew, the final piece is to make sure you are pitching plenty of healthy yeast to eat up all the sugars and create alcohol and other goodies. The current cheapest and easiest method to understand yeast concentration in beer is with a simple viability stain and a silver -coated microscope slide that was originally designed to count blood cells, hence the name hemocytometer. Below is my SOP for methylene blue viability staining and manual microscopic cell counting. Some of this information is for professional brewers only, but the counting and staining process can be applied to homebrew labs as well.
Standard Operating Procedure for the quantification of yeast cells and assessment of viability through the use of methylene blue staining
Purpose: To reliably complete fermenations to target final gravities, the first test any brewery can do for quality control of fermentation is monitoring of yeast viability and cell density through the use of the permeable dye methylene blue. This dye rapidly enters dead and injured yeast cells, while healthy cells with intact cell membranes are able to expel the dye. Harvesting and re-pitching yeast is a common practice in most breweries. Brewers should be able to reuse yeast for at least 7 generations and often as many as 10 generations if good harvesting and storage practices are followed. Harvesting and re-pitching yeast is a great way to spread the cost of the culture over many brews. Effective counting of yeast pitches also allows for correct pitching rates, in general:
Ales with a specific gravity < 16 °P: pitch 0.75 million cells/degree plato/ml. Ales with SG > 16°P: pitch 1-1.25 million cells/degree plato/ml Lagers with SG < 16 °P: pitch 2 million cells/degree plato/ml
Background and essential knowledge: Many brewers are afraid to look at yeast and assume any weight of yeast that “looks good” is enough to get by. Please take a moment to consider that yeast is not a black box, but it is actually a living organism with specific growth requirements. We need to consider the genetic and metabolic changes that occur during beer fermentation to fully consider proper yeast harvesting techniques.
Yeast cells that flocculate early tend to be less attenuative and will settle to the bottom of the tank with trub. Cells that stay in suspension and flocculate later tend to be more attenuative and will end up at the top of the yeast bed. Repeated selection of either of these extremes will change the profile of the culture and alter fermentation characteristics. Yeast to be harvested should be selected from the middle portion of the yeast bed when harvesting from the bottom of a tank or from the middle skim when harvesting from the top of a tank. It is usually a visible change from the first runnings of yeast which are darker to a nice creamy white buff color.
What Yeast Should Be Harvested:
Not all yeast in the brew house can be successfully re-pitched. When choosing a tank to harvest from and what culture to reuse, the following guidelines should be adhered to:
Yeast Generation: Always select the youngest generation of yeast available for harvest. Using fewer generations will minimize opportunities for mutation or contamination.
Previous Fermentation: Always harvest from a low gravity and low hopped beer. High gravity and/or highly hopped beers can stress the yeast and have detrimental effects on future fermentations. Do not harvest yeast from beers with alcohol contents greater than 6.5% ABV.
Yeast Evaluation: Only harvest yeast from fermentations that have exhibited normal fermentation characteristics. Always evaluate the yeast slurry as it is harvested. The slurry should appear thick and creamy with very little trub and no “off” flavors and aromas. Strong sulfur or phenolic aromas indicate possible problems with either sanitation or stress. Yeast should be tested for purity if possible and checked for viability and cell density. If there are any concerns over the health or purity of a culture, DO NOT USE IT!
When Yeast Should be Harvested:
The timing of cropping will have effects on the quality and density of the slurry. Consistent timing of harvest will help maintain the desired characteristics of the culture.
(Bottom Cropping): Yeast should be harvested once the temperature has dropped below 40 °F (4 °C) and trub has been discharged. This will insure a large yield of clean, homogeneous slurry.Yeast should be harvested from the same portion of the yeast bed each time. The harvested yeast should be light in color, creamy in texture, free of trub, and taste fresh with no off aromas. Harvesting from the middle of the yeast bed helps prevent selecting yeast that is either more or less flocculent than the previous generation.
How to harvest yeast:
Ensure there are clean and sanitized yeast brink kegs ready to go
Ensure there is a clean dump (T, sightglass, butterfly valve)
Ensure a yeast 3/16 ID hose with tri clamp ends is sanitized (PAA or pasteurization).
Slowly open the bottom butterfly valve of the fermentation vessel that is going to be harvested to see if there is a dry turd that blocks the draining of the yeast. You may have to pull the valve back and forth several times to loosen the turd. Be careful though because yeast spray can come out at any moment. It is useful to have a 5 gal bucket nearby to hold over the valve to catch any yeast spray. Once the yeast is flowing freely, spray out the butterfly valve with water and proceed to the next step.
Connect a dump to the bottom butterfly of the vessel to be harvested. Attach the braided yeast brink hose to the sight glass of the dump, and attach a hose to the butterfly valve. Run hot water through the dump to pasteurize the yeast hose (or PAA with cold water and 15 min contact time). Reach 180 degree F for at least 5 minutes. Allow yeast hose to cool to room temperature or attach yeast hose to cold yeast brink and allow sanitizer to run through yeast hose and dump to cool.
Attach a dump brewery hose to the sight glass of your dump. Run the yeast into the drain with some water from the wall running, until you get a nice creamy consistency. After you are happy with the color, attach yeast hose to the bottom of the fermentation vessel and the other end to the empty and sanitized yeast brink. Open the top butterfly valve of the yeast brink. Allow the yeast from the tank to flow into the brink slowly, be careful not to open the valve too quickly which can cause channeling in the yeast cone. If you go slowly, filling a yeast brink should take 20-30 minutes. Once the yeast brink is full (40-55 kg) place yeast brink in the cold room until use. Do not use yeast stored for more than 6 days.
Frequency of yeast cell counting and viability checks: During every yeast harvest (at least 1 yeast brink sample) and after every yeast pitch (full batch knockout sample from fermenter).
Equipment needed:100 ml plastic volumetric cylinder cleaned with water before and after
Plastic disposable 1 ml pasteur pipettes
Plastic screw cap poly bottles for sampling yeast and mixing with dye cleaned with water before and after
Gram increment scale
Microscope with 10X -40X objective
Hemocytometer
Glass coverslips
Materials needed: 1% v/v aqueous Methylene blue solution. This can be ordered from amazon.
100 g or 100 ml of yeast sample
100-200 ppm peracetic acid aqueous (PAA) sanitary solution in plastic spray bottle
Procedure
Yeast sample collectionIf sampling from yeast brink Shake the brink by rolling on the floor, for 1 min, until the yeast sample has homogenized, before sampling. This will likely build up pressure in the brink. To sample after homogenization, spray the bottom butterfly valve with PAA sanitary solution and open slowly to spray yeast slurry into disposable sampling cup. Allow the cup to fill, then dump the solution and refill. Aim for at least 100 g of yeast from a yeast brink, take the yeast sample into the lab. Alternatively, a sample can be taken directly from the fermentation vessel during yeast harvest, however it should only be taken this way if the tank has been crashed for 48 hr to allow for full flocculation of yeast.
If sampling from a fermenting beer, spray the sampling cock with sanitary solution and then open the sampling cock. Allow the solution to flow for 10-15 seconds and then collect 100 ml of fermenting beer in a plastic nalgene screw cap bottle. Screw the lid on the sample tight and shake to degas the sample.Take the yeast sample into the lab and degas it thoroughly by shaking it many times and burping the lid to release gas.
Yeast dilution and viability staining with methylene blue
Weigh out 98.5 ml of clean cold tap water in a clean 100 ml plastic volumetric cylinder.
Add 1 g of yeast slurry to the flask from the yeast sample with a plastic pasteur pipette.
Pour the mixed solution into a plastic poly bottle (labled blue) and stained blue. This is to avoid staining the volumetric cylinder.
Add 0.5 g of methylene blue dye to the plastic poly bottle and shake for 1 min.
Hemocytometer Preparation
Clean the Hemocytometer and glass cover slip with glass cleaner or tap water and wipe dry with a kimwipe. Make sure you clean and dry both the top and bottom of the slide.
Place a dry glass cover slip over the counting chambers.
Cell Counting ProcedurePipette 2-3 microliters of cell sample into the hemocytometer by using the 1 ml pasteur pipettes. It is not important what actual volume you add, just make sure that you let the capillary action of the glass cover slide move the liquid into the counting area and do not overflow the moats.
Wait 60 seconds for the cells to settle.
Manually Count Cells in Sample1. Place the hemocytometer under the microscope with the yellow 10x magnification objective. Turn on the light for the microscope, make sure the scope is plugged in first!Focus onto the grid pattern and the central 5 by 5 square that is outlined in triple thick lines and then switch to the higher magnification objective 40x (blue).
Count the number of total cells in each of the four corners of the 5 x 5 grid and the central square. Then count the number of blue stained cells (dead cells). Do not count cells which are conjoined and stained blue as dead, because these cells are undergoing cell division and are not considered dead.
If cells are touching the outside lines of the square, do not include them in the final count if they are touching the TOP or LEFT.
Write down the total number of cells from all 5 squares of both live and dead cells. Count the grid on each side of the hemocytometer.
Average your total counts from the two sides of the hemocytometer.
Divide the number of dead cells/total number of cells. Subtract this number from 100% to determine the viability of the sample. Average the % viable cells from both sides of the hemocytometer.
Example of 100 X magnification view with the 5 squares in green to be counted.
Example of how to exclude cells that are on the line.
Cell Calculations
1. If 5 squares of the central 5 x 5 grid were counted then multiply your total cell number by 5. If you counted all 25 cells in the central grid, then do not multiply the cell total.
2. If you diluted your sample by adding 1 g of yeast slurry into 100 ml of water/methylene blue then multiply the product of 1 by 100. If you diluted your sample by adding 50 ml of fermenting beer into 50 ml of water/methylene blue then multiply the product of 1 by 2.
3. Multiple the product 2 by 10,000 to account for the volume of the counting chamber. This will give you your total cell number per ml of sample.
Example: if you counted 323 total cells in 5 squares, and diluted your sample 1:100, then
323 yeast cells X 5 X 100 X 10,000 = 1,615,000,000 cells/g
Example: if you counted 323 total cells in 25 squares and diluted your sample 1:1, then
323 X 2 X 10,000 = 6,460,000 cells/g
Take the total cell density count and multiply by the calculated viability to determine working cell density.
Example: 1,000,000 cells/g X 90% viability = 900,000 viable cells / g
Use the cell / g to calculate how many kilograms of yeast slurry to pitch.
All data is recorded (cell density and viabilities) in fermentation logs and brew sheets.
Troubleshooting: Sometimes yeast cells appear to float on the slide. This can make counting challenging. The likely culprit is that your sample was not degassed enough, or that the slide was overloaded with sample. Try, try, try again! Sometimes yeast cells are too crowded to count or too few to be accurate. This means your dilution is not correct. If there are too many cells, try diluting your stained sample again (10 ml of stained cells into 90 ml tap water). If there are too few cells try taking more of your yeast sample for example 50 ml of yeast to 50 ml of water for 1:1 dilution. Aim for at least 75 cells per 5 squares counted. If you have any questions or trouble ask for help!
Conclusion: Yeast counting and viability staining is an essential part of the production process and can be accomplished within 15-20 minutes. Production staff should be trained on the correct usage of this test to make sure yeast harvesting procedures are consistent across the brewhouse.
Final Thoughts: The process of brewing beer has been accomplished for millenia, even before we knew what yeast was. Now, fortunately with a little know-how we can accurately predict how the biochemical process occurs and what factors we as brewers can manipulate to influence the final product. Maintaining accurate measuring devices is critical to your success. Make sure your pH meter and density measuring devices are calibrated! And finally, if you don’t write it down, what’s the point? Please keep detailed records of all data and join me in the pursuit of better beer. I hope you enjoyed learning about the science of brewing and cheers until next time!








Happy to see you back and posting!